In order to sign an informed consent, we are able to sign it electronically provided that we use the appropriate tools. Informed consent is a document that is typically signed by the medical team and the patient.
What is informed consent?
Informed consent is the formal medical procedure with the purpose of applying the principle of patient autonomy. That is, the obligation to respect patients as individuals and to honor their preferences in medical care.
In certain cases the consent is tacit and understood, as in the case of a physical examination by a doctor.For procedures involving significant health risks or those with alternatives involved, informed consent must be presented in writing and signed by the patient. It is a legal right of the patient and an obligation for health services (doctors, nurses, anaesthetists…).
Informed consent is regulated in Spain by Law 41/2002 and Law 14/2007 on Biomedical Research. Informed consents affect many areas and processes within a hospital, health centre or clinic. These documents usually have several pages covering information, risks and, due to the significant number of consents that are generated on a daily basis, this is certainly a starting point to go paperless.
How many signatures does an informed consent require?
Generally, the informed consent is a form where the patient’s personal data is collected, besides listing the different possible risks.
The patient must fill out and sign the form. Therefore, the informed consent has at least two signatories:
- The patient himself
- The health professionals
Signing informed consent digitally
Signing consents by healthcare professionals could se simply done by using a digital certificate.
For the patient this way of signing results much more complex, as it may be the case that the patient does not have a digital certificate or simply does not know how to use one.
All this leads to the use of the digitized signature, which is capable of capturing biometric information of the patient during the signing process so it can be then inserted into the document in electronic format. During this process, legal evidence is collected to ensure that electronically signed documents hold up in a court of law.
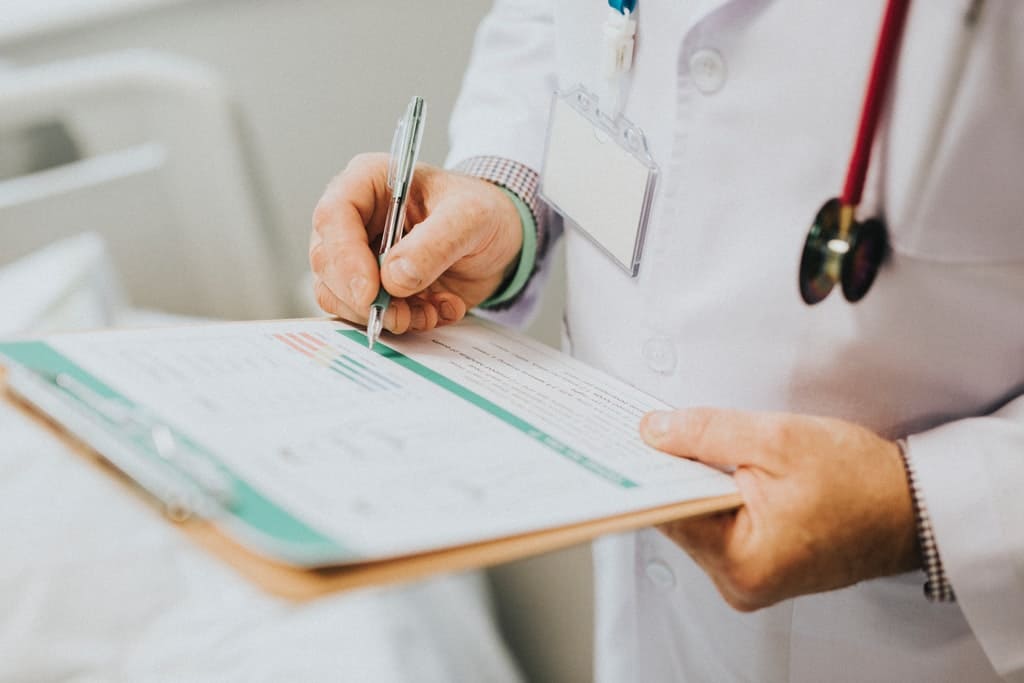
Informed consent signing can be done via Wacom or tablets which enable accurate electronic signature verification.
Among our success cases we find Laboratorio ROVI. Their professionals use our solutions to sign informed consents from their iPads.
Also, Ipsen Pharma Laboratories incorporated Viafirma’s solution for their sales process to sign consents via iPad using Adonit Jot Touch stylus with bluetooth connection.
If you are a developer or you are interested in integrating the electronic signature into your business processes, here we explain the steps to follow with an example.
Legal aspects of informed consent
In Spain, each Autonomous Community regulates their own Healthcare system as well as the type of device used to sign. However, above these regional laws, there is a Spanish legislation which stipulates that both electronic and digital signatures are legally valid. Want to learn more about the legal aspects and how GDPR affects consents?
